A Natural Psychedelic that May Help the Brain Heal
N,N-Dimethyltryptamine, (DMT), is a naturally occurring hallucinogenic tryptamine producing effects similar to those of other psychedelics like LSD, ketamine, psilocybin and psilocin. DMT occurs naturally in many plant species and animals including humans and has been used in religious ceremonies as a traditional spiritual medicine by indigenous peoples in the Amazon basin. DMT can also be synthesized in a laboratory.
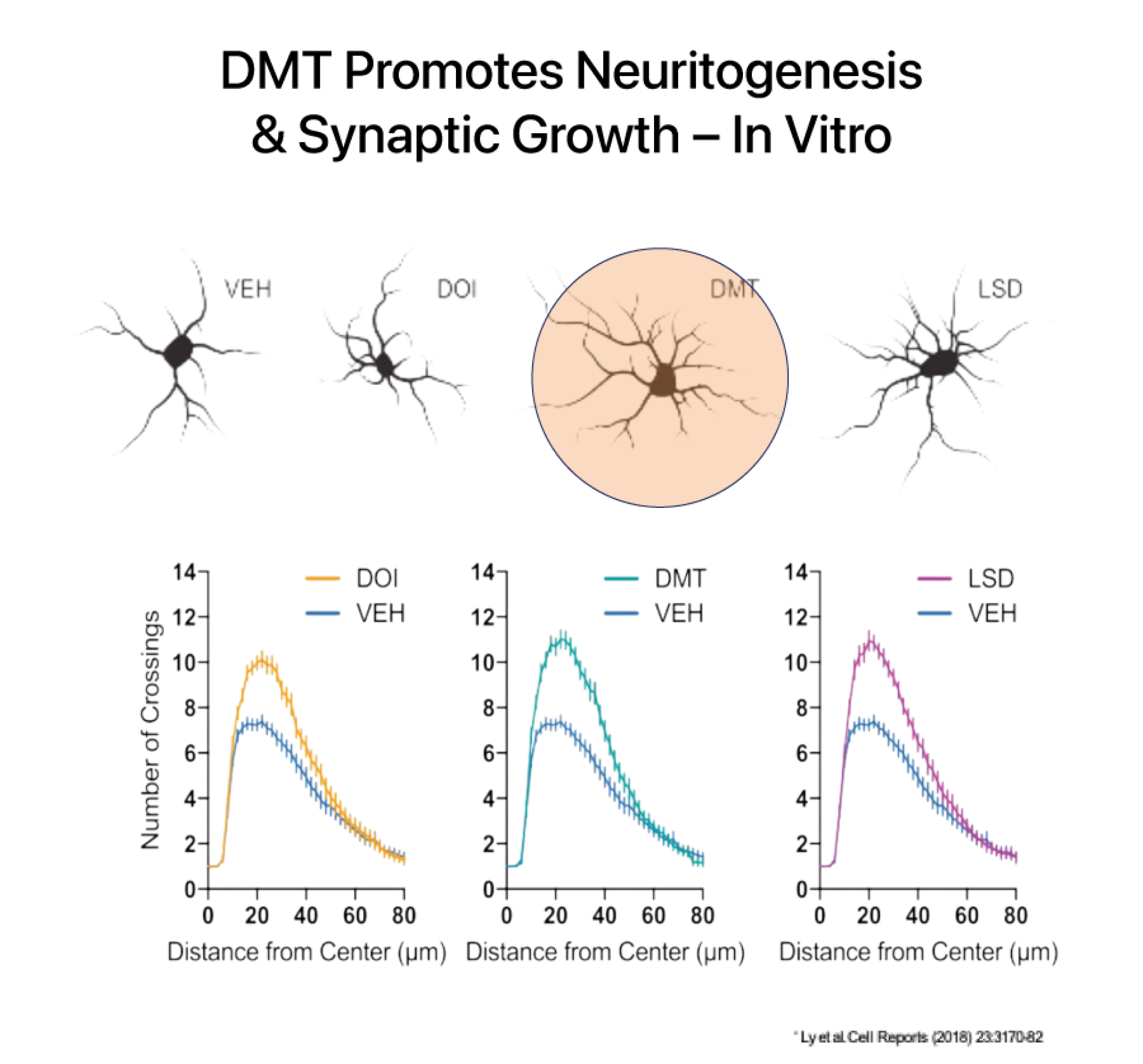
DMT has a rapid onset, intense psychedelic effects, and a relatively short duration of action at high doses. At sub-hallucinogenic doses, DMT has been shown to induce and improve structural and functional neuroplasticity both in vitro and in preclinical murine models.
DMT increases brain derived neurotropic factor (BDNF) which is a key mechanism involved in healing the brain after an injury like a stroke. DMT is believed to activate pathways involved in forming neuronal connections and has been shown to increase the number of dendritic spines on cortical neurons. Dendritic spines form synapses (connections) with other neurons and are a critical site of molecular activity in the brain.
A Phase 1 DMT trial is currently underway at the Centre for Human Drug Research in Leiden, The Netherlands. Following the completion of the study we will move forward and investigate an intravenous sub-hallucinogenic dose of DMT in acute ischemic stroke patients, in a planned Phase 2a DMT stroke study. A second Phase 2a study is being planned for traumatic brain injury (TBI).