Algernon Pharmaceuticals CEO To Deliver Virtual Keynote Presentation on Company’s DMT Program at the International Microdose DMT Conference
VANCOUVER, British Columbia, Sept. 09, 2021 (GLOBE NEWSWIRE) -- Algernon Pharmaceuticals Inc. (CSE: AGN) (FRANKFURT: AGW) (OTCQB: AGNPF) (the “Company” or “Algernon”) a clinical stage pharmaceutical development company, is pleased to announce that its CEO Christopher J. Moreau will be delivering the Keynote Presentation at the Microdose Virtual 2021 “The DMT Conference: A Molecular Masterclass”.
The conference will be taking place on September 9th and 10th and will bring together the world’s leading relevant experts in biotech, psychedelic research, as well as science and medicine. The conference will explore the potential of DMT related to drug development, safety, clinical care and applications, applicable law, regulation, business, markets, science, research, as well as history and culture.
Tickets are available for this important virtual event at Microdose Virtual 2021 DMT Conference Tickets
Moreau will be delivering his Keynote Presentation at 2:00PM EST on Thursday September 9th, which will be entitled “DMT – New Hope for Healing the Brain After a Stroke”. He will be providing a review of Algernon’s establishment of a unique clinical research program for the treatment of stroke focused on DMT, a known psychedelic compound that is part of the tryptamine family (other drugs in the tryptamine family include psilocybin and psilocin).
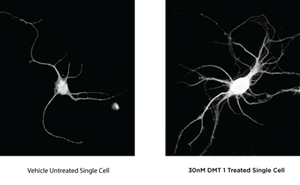
Algernon plans to be the first company globally to test DMT for stroke in humans and Moreau will be highlighting Algernon’s recent announcement that its own preclinical study has confirmed that DMT increased the growth of cortical neurons by 40% with statistical significance in one arm of the study, when compared to control. The Company also reported that the increased growth was achieved with a sub hallucinogenic dose.
The below images show the dramatic differences in neurite outgrowth comparing an untreated single cell on the left, with a single cell that was exposed to 30 nM DMT. The treated cell shows significant growth activity in the number of processes per cell.
About DMT
Algernon has established a clinical research program for the treatment of stroke focused on DMT, a known psychedelic compound that is part of the tryptamine family (other drugs in the tryptamine family include psilocybin and psilocin). Algernon plans to be the first company globally to test DMT for stroke in humans.
Algernon has also filed new provisional patents for new forms of DMT, in addition to formulation, dosage and method of use claims for ischemic stroke. The Company has also filed claims for combination therapy of DMT and Constraint Induced Movement Therapy (“CIMT”).
About Algernon Pharmaceuticals Inc.
Algernon is a drug re-purposing company that investigates safe, already approved drugs, and naturally occurring compounds, for new disease applications, moving them efficiently and safely into new human trials, developing new formulations and seeking new regulatory approvals in global markets. Algernon specifically investigates compounds that have never been approved in the U.S. or Europe to avoid off label prescription writing.
CONTACT INFORMATION
Christopher J. Moreau
CEO
Algernon Pharmaceuticals Inc.
604.398.4175 ext 701
info@algernonpharmaceuticals.com
investors@algernonpharmaceuticals.com
www.algernonpharmaceuticals.com.
Neither the Canadian Securities Exchange nor its Market Regulator (as that term is defined in the policies of the Canadian Securities Exchange) accepts responsibility for the adequacy or accuracy of this release.
CAUTIONARY DISCLAIMER STATEMENT: No Securities Exchange has reviewed nor accepts responsibility for the adequacy or accuracy of the content of this news release. This news release contains forward-looking statements relating to product development, licensing, commercialization and regulatory compliance issues and other statements that are not historical facts. Forward-looking statements are often identified by terms such as “will”, “may”, “should”, “anticipate”, “expects” and similar expressions. All statements other than statements of historical fact, included in this release are forward-looking statements that involve risks and uncertainties. There can be no assurance that such statements will prove to be accurate and actual results and future events could differ materially from those anticipated in such statements. Important factors that could cause actual results to differ materially from the Company’s expectations include the failure to satisfy the conditions of the relevant securities exchange(s) and other risks detailed from time to time in the filings made by the Company with securities regulations. The reader is cautioned that assumptions used in the preparation of any forward-looking information may prove to be incorrect. Events or circumstances may cause actual results to differ materially from those predicted, as a result of numerous known and unknown risks, uncertainties, and other factors, many of which are beyond the control of the Company. The reader is cautioned not to place undue reliance on any forward-looking information. Such information, although considered reasonable by management at the time of preparation, may prove to be incorrect and actual results may differ materially from those anticipated. Forward-looking statements contained in this news release are expressly qualified by this cautionary statement. The forward-looking statements contained in this news release are made as of the date of this news release and the Company will update or revise publicly any of the included forward-looking statements as expressly required by applicable law.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/9d6cd350-7a40-44ab-8ed6-a9da39d7f31e
 Source: Algernon Pharmaceuticals
Source: Algernon Pharmaceuticals
Released September 9, 2021